Biocon Gets USFDA Nod for Generic Anti-Cancer Drug
By Rediff Money Desk, NEWDELHI Feb 06, 2024 19:54
Biocon Ltd receives USFDA approval for Dasatinib tablets, a generic anti-cancer medication, strengthening its portfolio of complex drug products.
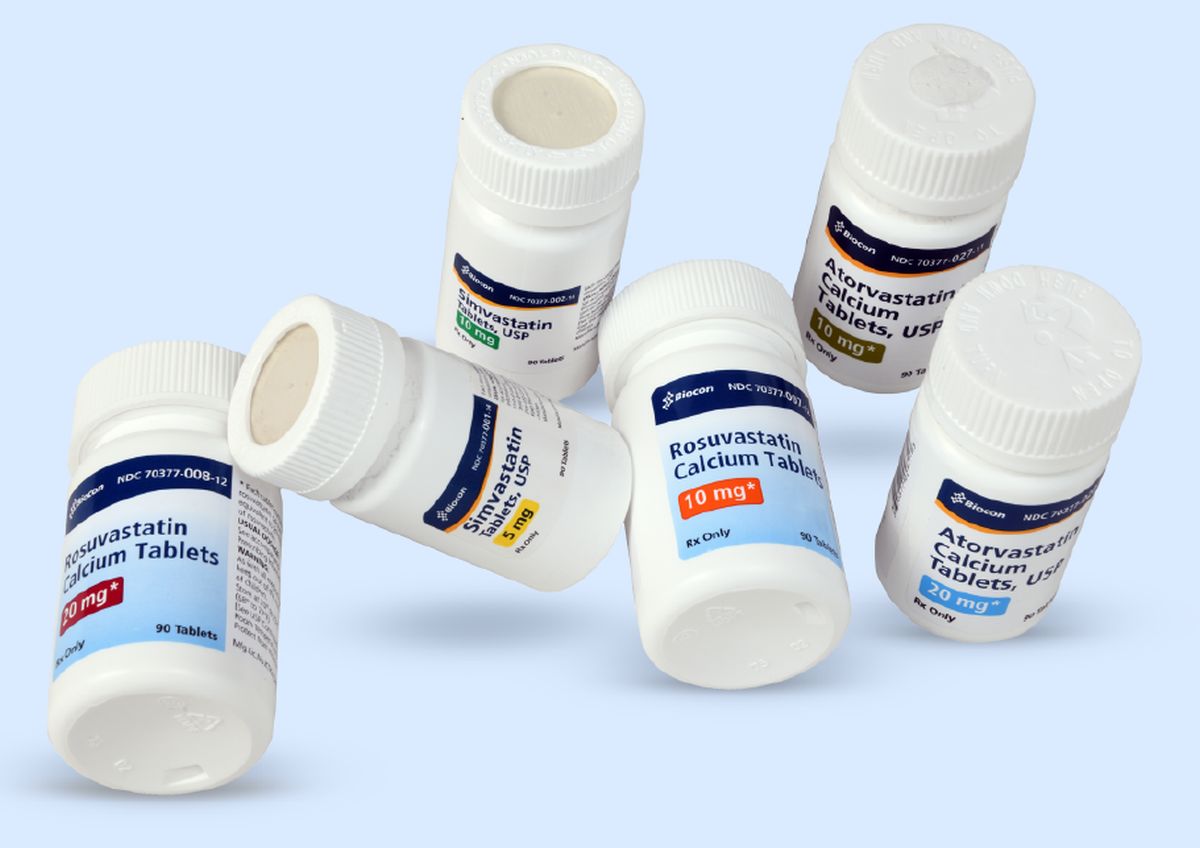
Photograph: Kind courtesy Biocon
New Delhi, Feb 6 (PTI) Biocon Ltd on Tuesday said it has received approval from the US health regulator for a generic anti-cancer medication.
The company has received approval from the US Food and Drug Administration (USFDA) for Dasatinib tablets to market in strengths of 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg, Biocon said in a regulatory filing.
The approval will further strengthen the company's portfolio of vertically integrated, complex drug products, it added.
Shares of Biocon settled 4.97 per cent higher at Rs 298.75 apiece on the BSE.
The company has received approval from the US Food and Drug Administration (USFDA) for Dasatinib tablets to market in strengths of 20 mg, 50 mg, 70 mg, 80 mg, 100 mg, and 140 mg, Biocon said in a regulatory filing.
The approval will further strengthen the company's portfolio of vertically integrated, complex drug products, it added.
Shares of Biocon settled 4.97 per cent higher at Rs 298.75 apiece on the BSE.
Read More On:
DISCLAIMER - This article is from a syndicated feed. The original source is responsible for accuracy, views & content ownership. Views expressed may not reflect those of rediff.com India Limited.
You May Like To Read
TODAY'S MOST TRADED COMPANIES
- Company Name
- Price
- Volume
- IFL Enterprises
- 1.36 (+ 4.62)
- 139837616
- Franklin Industries
- 3.90 (+ 4.56)
- 41704768
- Vodafone Idea L
- 16.28 ( -3.04)
- 37101580
- Sawaca Business Mach
- 0.67 ( -4.29)
- 36642255
- Tata Teleservices (M
- 97.43 (+ 19.80)
- 21113173
MORE NEWS

Sensex Hits 81,000, Nifty at Record 24,800: IT...
The Indian stock market continued its record-breaking run, with Sensex crossing 81,000...

World's Largest Coal Mines: India's SECL Holds...
Two coal blocks in India's South Eastern Coalfields (SECL) rank among the world's 10...

Infosys Q1 Profit Up 7.1%, Raises FY25 Outlook...
Infosys reported a 7% rise in Q1 net profit and raised its FY25 revenue growth guidance...







 © 2024 Rediff.com India Limited. All rights reserved.
© 2024 Rediff.com India Limited. All rights reserved.